Oculoplasty Evaluation with Botox Injections:
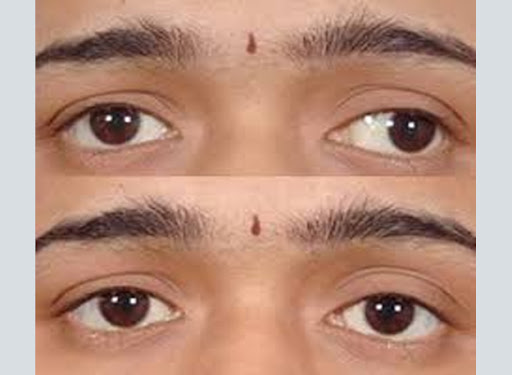
Botulism is a serious illness caused by the toxin of the bacterium Clostridium botulinum that may cause varying degrees of self-limited paralysis of the facial musculature and extremities to life threatening respiratory failure. The toxin can be acquired via ingestion from contaminated food, systemic absorption from infected wounds, or intestinal colonization in infants and adults. Botulinum toxin is also a feared mechanism of bioterrorism, although doses of 0.09-0.15 µg would be required to poison a human being.[1] These levels are approximately 100 times higher than the quantity of botulinum toxin found in one 100 unit vial of Botox® (0.00073 µg). In clinical uses of botulinum toxin in ophthalmology, treatments are FDA-approved and have time-tested favorable safety profiles.
History
The ubiquitous gram-positive anaerobe Clostridium botulinum was first identified as the cause of botulism in 1895 by the Belgian professor Emile Pierre van Ermengem.[2] During the following two decades, numerous other strains of botulinum toxin were isolated and identified. Dr. Herman Sommer produced a crude form of botulinum toxin A at the University of California, San Francisco in 1926 and Dr. Edward Schantz and his colleagues first produced a purified form of botulinum toxin A in 1946. [3] [4] In the 1970s, the first clinical application of botulinum toxin was pioneered by Dr. Alan Scott, an ophthalmologist, who injected botulinum toxin in human extraocular muscles to treat strabismus.[5] Over the past 2 decades, the therapeutic applications of botulinum toxin have greatly expanded to include the treatment of various disorders of muscle hyperactivity, hyperdynamic rhytides, hyperhidrosis and migraine headaches.
Mechanism of Action
The mechanism of action of botulinum toxins utilized in oculofacial plastic surgery involves inhibition of acetylcholine release at the neuromuscular junction, which results in paralysis/paresis of muscle tissue.[6] The toxin is a single 150 kilodalton molecule composed of a heavy and light chain. The heavy chain binds to specific receptors on motor nerve terminals, which subsequently internalize the toxin via receptor-mediated endocytosis. Within these endosomes, the toxin is subjected to an acidic pH around 4-5 which is believed to result in dissociation of the light chain from the toxin and passage of the light chain into the cytosol of the motor neuron. Within the cytosol, the light chain is then able to cleave a specific protein responsible for acetylcholine vesicle exocytosis. These proteins, referred to as the SNARE complex, consist of: 1) synaptosomal associated protein (SNAP-25), 2) vesicle associated membrane protein (VAMP or synaptobrevin); and 3) syntaxin, a docking protein within the synaptic membrane. Botulinum toxin A is known to cleave SNAP-25, and toxin B cleaves synaptobrevin.
Clinical Applications
The most common oculoplastic applications for botulinum toxin include: benign essential blepharospasm, hemifacial spasm, hyperfunctional facial rhytides, and strabismus. Treatment with botulinum toxin is both safe and effective[7][8][9]; therefore, it is often used as first line treatment in debilitating myoclonic disorders. In addition, hypersecretion of the lacrimal gland secondary aberrant regeneration of the facial nerve can also be successfully treated with botulinum toxin. The mechanism involves inhibition of acetylcholine release from postganglionic parasympathetic nerve endings that supply lacrimal gland acini. Patients with other types of hypersecretion such as hyperhidrosis, gustatory sweating (Frey syndrome), and sialorrhea have also shown clinical improvement from local injections of botulinum toxin.
